Metastatic cancer can be treated with drugs in the future
Metastatic cancer is in many cases incurable, and the treatment of advanced cancer remains an unresolved problem for the field of medicine. The development of effective treatments requires profound understanding of the ways metastases are formed in different tissues.Published 4.4.2024
Text: Mitro Miihkinen
Image: Shutterstock
Editing: Viestintätoimisto Jokiranta Oy
Cancer is a collection of diseases in which normal tissue cells become malignant and begin to proliferate in an uncontrolled manner. Of the various characteristics of cancer diseases, the most severe is the formation of metastases, through which the cancer spreads from its original site to other tissues. Today, effective treatments are increasingly available to tackle different types of cancer, but the treatment of advanced metastatic cancer still remains an unresolved problem in medicine.
The spreading of cancer varies individually and it depends on a number of factors, including the type of cancer. Some cancers, such as basal cell carcinoma within the skin, hardly ever transform into a metastatic disease. On the other hand, pancreatic cancer is an example of an efficiently spreading cancer that often has metastases already when initially diagnosed. The rest of cancers fall between these two types in terms of the formation of metastases. In breast cancer, for example, 30 per cent of patients are affected by a metastatic form of disease later in their life, sometimes several decades beyond the initial phase.
In the future, we may live in a world where metastases can be effectively cured through medication. With few exceptions, this is not the case for the time being, and that’s why we need basic research aimed at developing medicinal treatments for metastases.
A cancer cell is highly adaptive
Cancer diseases are named on the basis of the tissue or organ in which the disease starts. Breast cancer, for example, has its origin in the breast tissue. The pathogenic cancer cells may, however, begin to move actively within the tissue of origin and migrate into, for example, blood vessels. Through the bloodstream, they will end to other tissues, such as the lungs.
In the lungs, the migrated cancer cell may not be able to grow since it is not accustomed to the environment offered by the new tissue type. Thus, fortunately, the majority of cancer cells will never form metastases. The reverse side of the coin is, however, that a metastasis only needs a single cell to start growing.
The formation of metastases can also be approached through the theory of species evolution. Evolution refers to adaptive changes in species due to environmental pressure, such as the availability of nutrition or threat of predators. Through these adaptive changes, the species evolve to survive in a particular environment. A major adaptation may lead to speciation, that is, the genesis of new species with characteristics and behaviours that distinguish them from the original species.
The concept of evolution can be applied to metastases and their formation process: on its way from the primary tumour to a metastasic site, the cancer cell encounters several challenges that call for adaptation. Such challenges include, for example, the immune defence of the body or the availability of resources like nutrients.
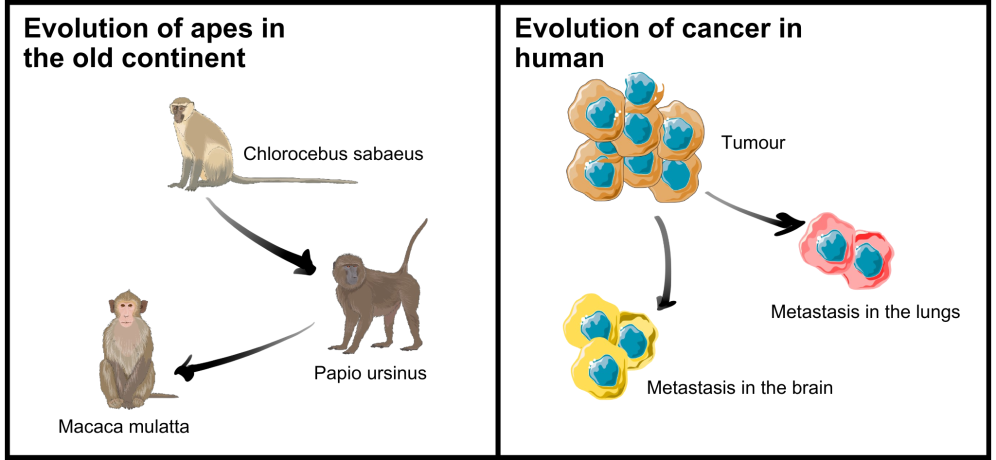
Similar to other organisms, apes are the result of speciation. The spreading of cancers can also be approached through evolutionary theory and phylogenetics.
Drug development calls for basic research
How different are the metastatic cells from the original cells? Does the journey from the primary tumour to a metastasic site involve speciation? The answer is probably ‘yes’, since metastases rarely respond to those cancer drugs that are highly efficient against the original tumour cells. Evidence has been presented to support this idea, by showing, for example, that gene expression is different between the metastases and the original tumour. The million-dollar question is how this different gene expression facilitates the growth of metastases and whether the growth could be slowed by means of new drug molecules.
The Sakari Alhopuro Foundation provides funding for basic research aimed at understanding metastatic growth mechanisms. The ultimate goal is a healthier world and provision of successful treatment for patients with metastatic cancers. To make this happen, we need long-term basic research and better understanding of the growth mechanisms of metastases in different tissues.

Mitro Miihkinen works as a postdoctoral researcher in the Computational Systems Medicine group led by Dr. Tero Aittokallio within the Institute for Molecular Medicine Finland (FIMM) at the University of Helsinki. Miihkinen earned his PhD in cell biology and drug development from the University of Turku in 2021. Since then, he has been dedicated to research on the growth of metastases by means of combined experimental and computational methods. His research will add to the knowledge of metastatic activities and potential medication, while also developing new computational-experimental tools for the open-access use of other researchers.
